Energy Bands in Solids
1.1 Introduction:
The property of solids that varies most from one solid to the other is the ability to conduct electric current. For example, the resistivity of copper is 1.7 ✕ 10 ^ -8 Ω-m whereas that of quartz is 7.5 ✕ 10 ^ 17 Ω-m. The electron energy bands present in the solids makes it possible to study such a wide variation in resistivity and conductivity.
1.2 Structure of an Atom
Any substance, solid, liquid or gaseous is made up of molecules and molecules and molecules are made up of atoms. The atoms contains tiny particles called protons, neutrons and electrons which are called as fundamental particles. Protons have positive charge, electrons have negative charge and neutrons are electrically neutral. The protons, electrons and neutrons of various types of atoms ( atoms of different materials) are same as each other. That means there is no change in their physical properties though they are present in different materials. But the "arrangement" of protons, neutrons and electrons we be different in different materials. Figure 1 shows that an atom consists of a central "nucleus" which is surrounded by orbiting electrons.
Figure :- 1
Above shown figure shows that an atom consists of a central "nucleus" which is surrounded by orbiting electrons.
We can compare this structure of an atom to our solar system in which sun is at the center and planets revolve around it in fixed "orbits".
From the above figure of structure of atoms we can study that,
1) The Nucleus : The nucleus of an atoms consists of two types of particles a) Protons and b) Neutrons. In an given atom, number of protons are equal to the number of revolving electrons outside in the shell
2) Atoms are electrically neutral : Since the protons and orbital electrons are equal in number, their equal and opposite charge will neutralize each other electrically neutral.
3) Atoms can be converted to ions : If an atom loses an electron then the number of protons becomes higher than the number of electrons. Therefore the atom becomes positively charged and it is referred to as a Cation or positive ion. Similarly if an atom gains an additional electron then it becomes negatively charged and called as Anion or negative ion.
Figure :- 2
4) Electron orbits or shells : It has been discovered that the electrons can occupy only certain orbital rings or shells which are at a fixed distance from the nucleus. Each shell can contain only a particular number of electrons. In general a shell can contain at most at the most 2 n^2 number of electrons where "n" is the shell number. For example, the first shell can contain upto 8 electrons and so on. The exception for this rule is that the outermost shell cannot contain more than eight electrons.
5) Valence shell and valence electrons : The outermost shell is known as the valence shell and the electrons in it are called as valence electrons. These valence electrons determine the electrical ( and chemical) characteristics of each particular atom. The valence shell may be completely filled or partially filled of valence electrons.
6) Energy levels of electron shells : The closer an electron is to the nucleus, the stronger is the force that binds it. Each shell has an energy level represents the amount of energy that is required to be supplied to extract an electron from the shell. As the valence electrons are the farthest from nucleus, it requires the least amount of energy to extract them from the valence shell. On the other hand the electrons which are in the innermost shell will require maximum amount of energy for their extraction from the atom.
7) Energy level and electron volt (eV) : The energy levels considered above are measured in electron volt (eV). An electron volt is defined as the amount of energy required to move one electron through a potential difference of one volt. The charge on one electron is 1.6 x 10 coulomb. So the energy required by an electron to fall through a potential of 1 volt is called as one electron volt.
∴ 1 eV = 1.6 x 10 ^ -19 J x 1V
∴ 1 ev = 1.6 x 10 ^ -19 J
8) Effect of additional energy absorbed by an atom : An atom can absorb additional energy due to increase in temperature, or from the light focused on it etc. Due to this absorbed energy, the energy levels of the electrons are raised, and they move to the higher orbits i.e. the orbits which are away from the nucleus. If energy is given to the valence electrons then they will jump out of the valence shell and become "free". Such free electrons are not bound to the nucleus of any atom. These "free electrons" constitute the flow of current through a material.
Atomic number
The number of protons in an atom is referred to as the atomic number of the atom. The atom of "silicon" has 14 protons and 14 neutrons in its nucleus therefore the atomic number of silicon is 14.
Atomic weight
Atomic weight is approximately equal to the total number of protons and neutrons in the nucleus of the atom. Thus the atomic weight of the silicon atom is 28.
1.3 Concept of Energy Level
Each electron orbit has an energy level associated with it. The electrons in the inner orbits are more closely bound to the nucleus and posses less energy. But as we move towards the valence shell, the binding force between nucleus and electrons reduces and the electrons posses higher energy. The energy of shell-1 is the lowest and that of the valence shell is the highest. The concept of energy levels is illustrated in Figure 3 and Figure 4
Figure :- 3
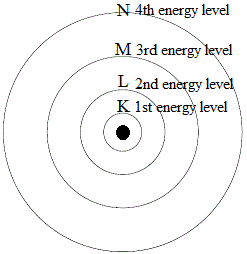
Figure :- 4
Free electrons
The valence electrons are very loosely bound with the nucleus. If an external energy is given to them,then they can easily break away from the nucleus and become free. Such electrons which are free from the force of attraction of nucleus are called as free electrons. These free electrons posses an energy which is higher than that of valence electrons. The electric current flows due to these free electrons and they are said to be in the conduction band.The energy level of conduction band is higher than that of valence shell.
1.3.1 Formation of Energy Bands (Band Theory) in Solids
- In the preceding section we have learnt that along with every electron shell there is an associated energy level.
- The electrons in the first shell will require the highest amount of energy for their extraction. Therefore the first shell is said to have the lowest amount of energy associated with it.
- On the other hand, the valence electrons require the lowest amount of energy for their extraction. Hence valence shells are said to have the highest amount of energy. This discussion is concerned with one single isolated atom.
- The electrons in the first shell of every atom will have energy levels which are close to each other but not exactly same. There will be a slight difference in the energy levels of electrons in the first shell of different atoms.
- In practice, the atoms are not isolated from each other. When they are brought together the electrons will be acted upon by the forces from other atoms.
- Under such circumstances, the energy levels occupied by the electrons will get merged to form energy bands as shown in the figure. 5.

- The first band in Figure 5 is formed by cluster (bunch) of energy levels of electrons in the first shell of millions of atoms. Similarly the other bands shown in Figure 5 get construed.
- The energy band diagram shown in Figure 5 is simply graphical representation of the energy levels associated with electrons in different shells of atoms in a solid.
Valence band
The valence band corresponds to the valence electrons present in different atoms of the materials. Energy associated with the valence band is the second highest as shown in Figure 5.
Conduction band
Conduction band has the highest energy associated with it as shown in Figure 5. The electrons in the conduction band are the "free" electrons i.e. the electrons which are disconnected from their respective atoms.
Conduction band electrons are actually responsible for the flow of current. More the number of electrons in the conduction band more will be the current flow.
1.3.2 Forbidden Energy Gap
As shown in Figure 5, the forbidden gap is the energy gap that separates the conduction and valence bands. No electrons can normally exist in the forbidden gap. For any given type of material the forbidden gap may be large, small or even nonexistent. The materials are classified as conductors, insulators and semiconductors based on the relative widths of the forbidden gap.
Jump from valence band to conduction band
If the valence band electrons can jump across the forbidden gap and enter into the conduction band then they will become free electrons and be available for conduction. The valence electrons can jump if we provide additional energy to them. This additional energy can be supplied by increasing the temperature or focusing light on the material etc. This is the reason why conductivity of certain materials increase with increase in temperature.





Comments
Post a Comment